The SALSA MLPA Probemix ME030 BWS/RSS is a research use only (RUO) assay for the detection of aberrant methylation of one or more sequences of the following differentially methylated regions (DMRs): KCNQ1OT1:TSS-DMR (also called IC2), H19/IGF2:IG-DMR (also called IC1) in the 11p15 chromosomal region associated with Beckwith-Wiedemann syndrome (BWS) and Russell-Silver syndrome (RSS). This probemix also includes probes for the IGF2:alt-TSS-DMR in the 11p15 chromosomal region. Additionally, this assay can be used for the detection of aberrant methylation of one or more sequences of the MEST:alt-TSS-DMR and GRB10:alt-TSS-DMR on chromosome 7 associated with RSS. This probemix can also be used to detect deletions/duplications in the aforementioned chromosomal regions. For better coverage of chromosome 7 and chromosome 14, we recommend to use SALSA MLPA Probemix ME032 UPD7-UPD14.
Genomic imprinting is the monoallelic expression of genes, dependent on the parental origin of the chromosome. It plays a role in growth and development. Imprinting disorders originate from a disturbance in this monoallelic expression by disruption or epimutation of imprinted genes (Ishida et al. 2013).
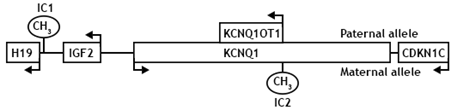
BWS is a clinically heterogeneous overgrowth syndrome associated with an increased risk for embryonal tumour development. RSS is a genetically heterogeneous disorder involving both intrauterine and postnatal growth retardation. The incidence of both BWS and RSS is estimated to be approximately 1 in 10,000-15,000 newborns and around 85% of the cases are sporadic (Õunap 2016). These conditions are both caused by a genetic or epigenetic alteration within two domains of imprinted growth regulatory genes on chromosomal region 11p15, leading to deregulated expression of the imprinted genes within this region. Approximately 60-70% of the patients have imprinting abnormalities at one of two imprinted domains IC1 or IC2, and these changes are frequently mosaic (see Figure 1 for a scheme of the imprinted gene cluster). Other known causes of BWS and RSS are uniparental disomy (UPD), trisomy 11p15, mutations in the CDKN1C gene, as well as small deletions and translocations. About 10% of RSS cases are caused by maternal UPD for chromosome 7 (Õunap 2016).
This SALSA MLPA Probemix ME030 BWS/RSS is capable of rapidly detecting most causes of BWS and RSS, as both copy numbers and methylation status of the 11p15 region can be determined. This MS-MLPA assay for BWS/RSS can also be useful for screening of childhood cancers, in particular Wilms' tumour. A strong linkage between hypermethylation of the IC1 locus, but not IC2, has been described in these patients resulting in biallelic expression of the IGF2 gene (Maas et al. 2016).
More information is available at https://www.ncbi.nlm.nih.gov/books/NBK1394/ (BWS) and https://www.ncbi.nlm.nih.gov/books/NBK1324/ (RSS).