News Archive
New Product: NXtec D028 Carrier Panel 1
We are proud to introduce NXtec D028 Carrier Panel 1 - a high-throughput assay detecting CNVs and selected SNVs for carrier status analysis. Read more.
Our Products are IVDR-Certified!
We have successfully obtained our certification under the new In Vitro Diagnostic Regulation (IVDR; 2017/746)! The first batch of flagship products are already available on the market under the new IVDR, well in advance of the transition period deadline. Read more.
A Year in Review – Reflecting on 2025
As we look back on 2025, we are excited to share some of the year’s highlights with you! Read more.
Prenatal Samples and Methylation Patterns
Several studies highlight the challenges inherent to using prenatal samples for methylation analysis. Therefore, we do not recommend using MS-MLPA probemixes on prenatal samples. Read more.
Expanding Access to Newborn Screening with MRC Holland's Melt Assay for Spinal Muscular Atrophy
MRC Holland's Melt Assay for SMA screening in newborns addresses the need for easy‑to‑adopt first‑tier screening technologies as more countries move toward implementing national newborn screening programs. Read more.
The Status of DNA‑Based Technologies in Newborn Screening
A recent publication by MRC Holland researchers contributes valuable insights into the evolving field of newborn screening (NBS). Read more.
Changes to Our Product Packaging
Our product packaging will receive updates which simplify processing and pave the way for future innovations – there are no changes to the product contents.
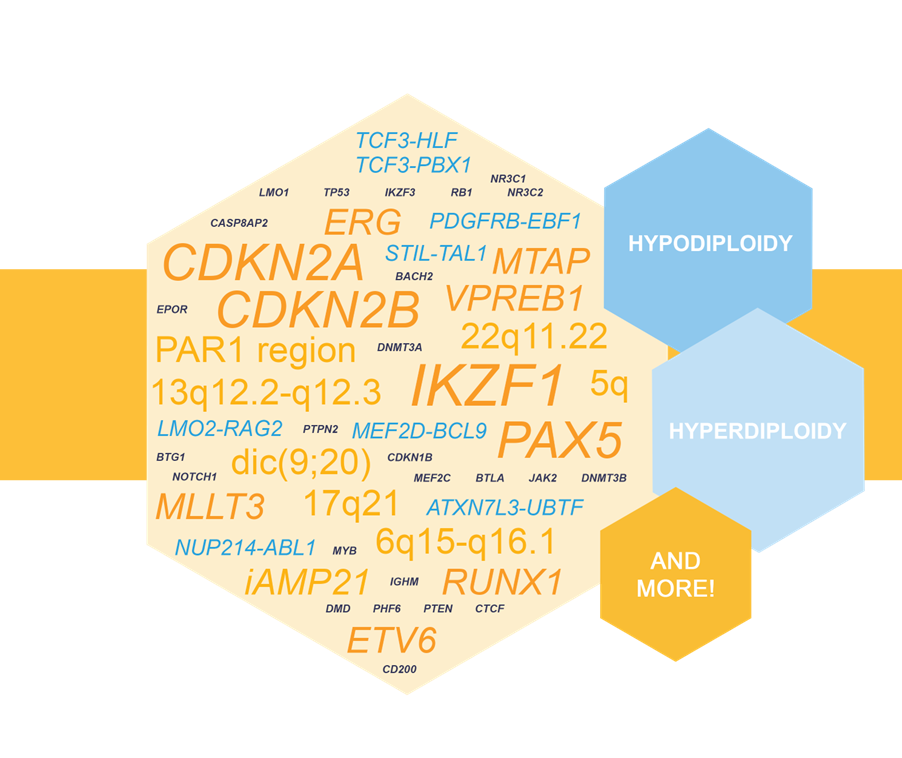
NXtec D007 Acute Lymphoblastic Leukemia as a Valuable Tool in pALL Testing
D007 Acute Lymphoblastic Leukemia was recognized as a valuable tool for enhanced molecular testing of pediatric acute lympoblastic leukemia (pALL) in a recent research article. Read more.
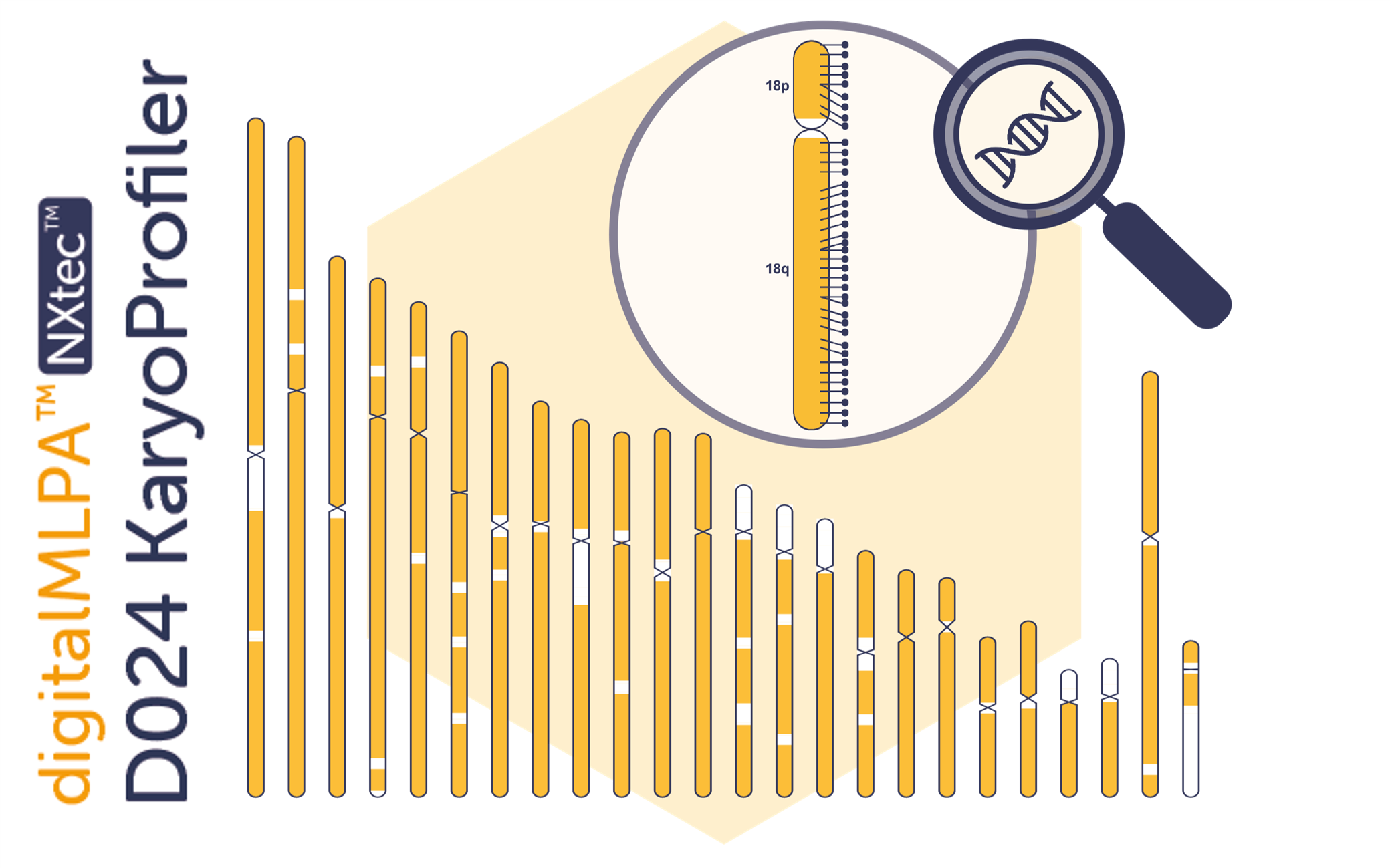
New Product: NXtec D024 KaryoProfiler — High-Throughput, High-Resolution Alternative to Traditional Karyotyping
We have launched NXtec D024 KaryoProfiler, our newest digitalMLPA™ assay designed for comprehensive molecular karyotyping across a wide range of applications. Read more.
New Product Launch: NXtec D002 Hereditary Cancer Panel 2 for Broad Hereditary Cancer Analysis
Introducing NXtec Hereditary Cancer Panel 2 – a broad panel for the detection of CNVs in cancer-associated genes and the presence of selected variants.