NXtec D007 Acute Lymphoblastic Leukemia as a Valuable Tool in pALL Testing
Our digitalMLPA™ assay NXtec™ D007 Acute Lymphoblastic Leukemia was recognised as a valuable tool for enhanced molecular testing of pediatric acute lymphoblastic leukemia (pALL) in an article recently published by researchers from the La Fe University and Polytechnic Hospital in Spain.
The study, published in Nature’s British Journal of Cancer, set out to benchmark emerging genomic approaches and standard of care methods in pALL, the most common childhood cancer. digitalMLPA, RNA sequencing, targeted sequencing, and optical genome mapping (OGM) were assessed on how they perform individually and in combination to deliver a more complete picture of the 60 pALL samples analysed.
The main findings showed that:
- standard-of-care methods alone identified disease-relevant markers in less than half of cases (~47%)
- modern genomic approaches improved the detection rates and subtype classification
- the combination of digitalMLPA + RNAseq outperformed all other methods, including OGM, detecting ~95% of markers
The authors describe the digitalMLPA + RNAseq combination approach as an emerging frontline strategy to make informed decisions and streamline the molecular testing of pediatric ALL. This combined approach minimises cascade testing, helps reduce costs and shortens turnaround times – thus meeting the real-world demands of ALL laboratories.
Read the full article here.
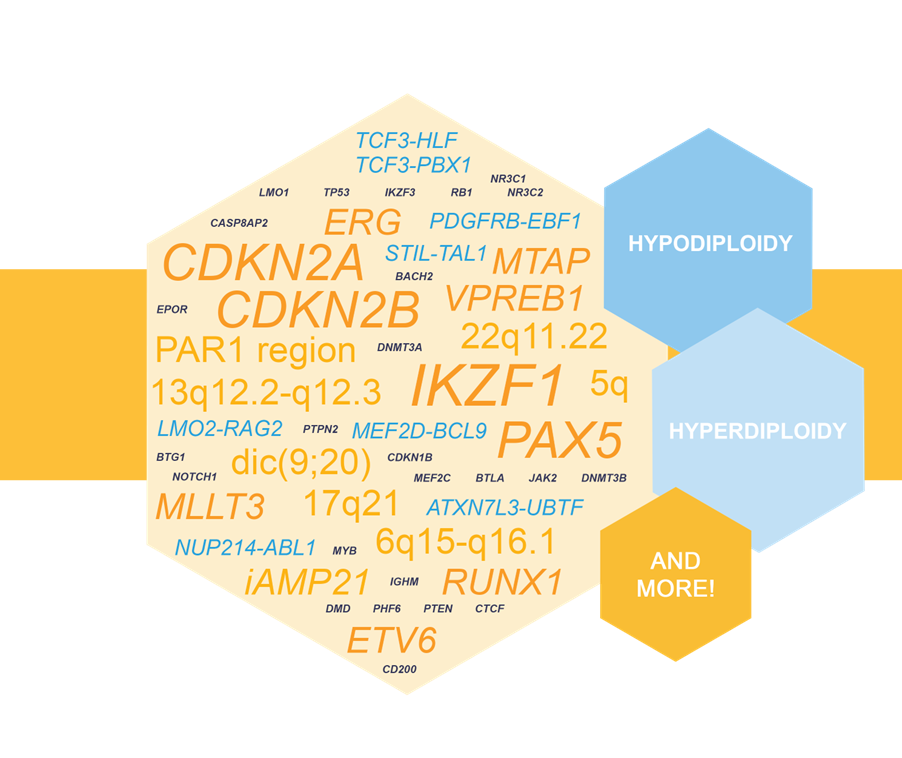
Key features of NXtec D007 Acute Lymphoblastic Leukemia:
- High-resolution coverage of 73 ALL-related genes and 8 regions including IKZF1, ERG, PAX5, iAMP21, PAR1 region, and many more.
- Includes 250 probes across the genome for gross copy number detection.
- Displays a high dynamic range for CNA detection.
Advance your pALL testing with a proven solution
Interested in trying out D007 Acute Lymphoblastic Leukemia? Contact us at info@mrcholland.com to request a personalized offer.